In early February, manufacturer Colgate-Palmolive recalled a popular household cleaning product called Fabuloso due to the risk of bacteria exposure.
Now, some news headlines, including this one from the Epoch Times, are warning of an eye product recall over “deadly bacteria.”
Recent online searches also show people are looking for information on eye ointment and eye drop recalls.
THE QUESTION
Have eye products been recalled due to a risk of bacteria exposure?
THE SOURCES
THE ANSWER
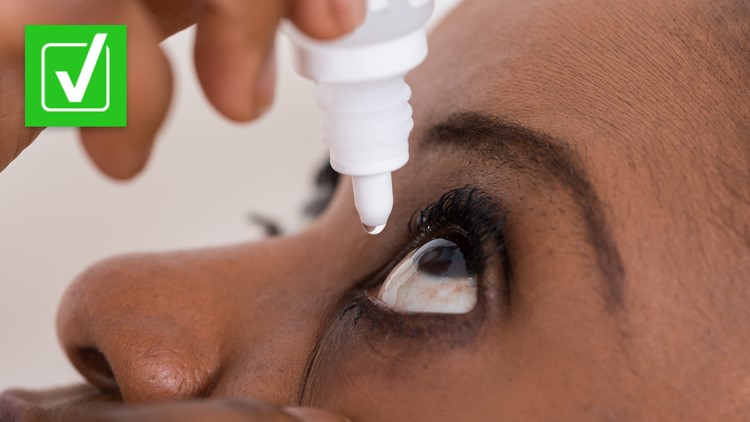
Yes, some artificial tear drop and eye ointment products have been recalled due to a risk of bacteria exposure.
WHAT WE FOUND
Artificial tear drops and eye ointments manufactured by the company Global Pharma Healthcare have been recalled because they could be contaminated with a rare, drug-resistant bacteria, according to the U.S. Food and Drug Administration.
Federal health officials are investigating an outbreak of that bacteria tied to artificial tear drops that has led to hospitalizations, blindness and three deaths.
On Feb. 21, the FDA said people should not purchase or use Delsam Pharma’s Artificial Eye Ointment due to potential bacteria contamination. One day later, Global Pharma agreed to recall the ointment.
In early February, the FDA warned people to stop using EzriCare and Delsam Pharma’s Artificial Tears due to bacterial contamination risk. There is an increased risk of eye infections that could result in blindness or death for people who use contaminated drops, according to the federal health agency.
Global Pharma voluntarily recalled all unexpired lots of the artificial tear drops after a recommendation from the FDA due to the company’s violations of manufacturing standards. Those violations include a lack of appropriate microbial testing and formulation issues, among others, the FDA said.
More from VERIFY: Yes, there is a recall on Fabuloso cleaner due to bacteria exposure risk
The CDC is currently partnering with the FDA and health departments across the country to investigate a multistate outbreak of the “rare” and “extensively drug-resistant” bacteria called Pseudomona aeruginosa associated with some of the recalled products. This bacteria strain had never been reported in the U.S. prior to the outbreak.
The number of deaths tied to the outbreak rose from one in late February to three as of March 14. Eight people have lost their vision and four have had their eyeballs surgically removed, according to the CDC.
Most of the patients reported using artificial tears, with the most common brand being EzriCare.
Lab testing by the CDC identified the presence of bacteria in opened EzriCare bottles from multiple lots. They were collected from patients with and without eye infections in two states.
The CDC says testing of unopened EzriCare bottles is ongoing.
Anyone who has used EzriCare or Delsam Pharma’s artificial tears and has symptoms of an eye infection should seek medical care immediately, the CDC says.
Symptoms of an eye infection may include yellow, green or clear discharge from the eye; eye pain or discomfort; redness of the eye or eyelid; the feeling of something in your eye; increased light sensitivity; and blurry vision.