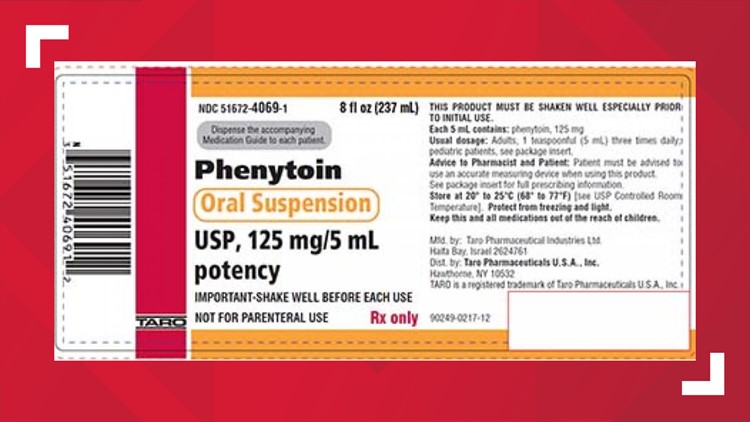
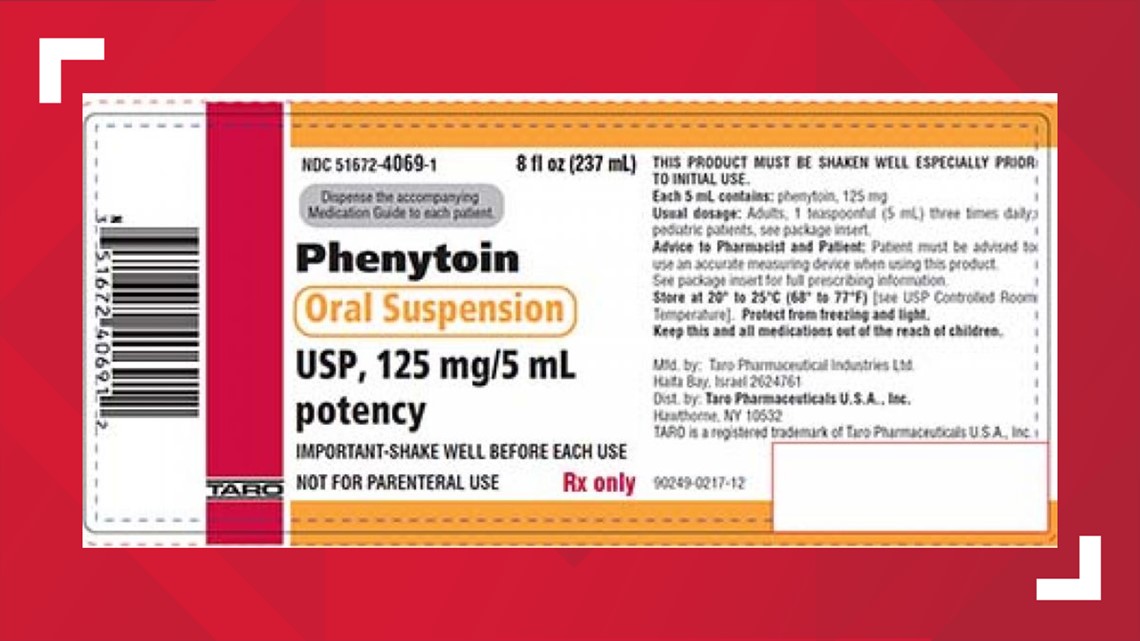
HAWTHORNE, N.Y. — The pharmaceutical company Taro voluntarily recalled two lots of seizure medicine, Phenytoin Oral Suspension USP, because the product may not re-suspend when shaken, which could cause under or overdosing.
The FDA said the drug is used to treat tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures. Young children and infants are primarily at risk.
The two lots that are being recalled are as follows:
- Lot #: 327874 Expiration Date: December 2020
- Lot #: 327876 Expiration Date: December 2020
The FDA said the labels show the name of the product, Phenytoin Oral Suspension USP, 125 mg/5 mL and the NDC #51672-4069-1. To date, Taro has not received any adverse event reports related to the recall.
Lot 327874 was distributed to wholesale distributors, long-term care providers, a repackager and mail-order customers in the U.S. market between May 3 and July 5, 2019.

Lot 327876 was distributed to wholesale distributors, long-term care providers and mail-order customers in the U.S. market between July 1 and August 21, 2019.
The FDA said the medicine may have further distributed the lots to retail pharmacies for prescription dispensing to patients who were prescribed Phenytoin Oral Suspension.
Taro is notifying its distributors and retail customers by phone, e-mail, and letters via U.S. Mail and is arranging for return of any containers or quantities of Phenytoin Oral Suspension Lots # 327874 and 327876. Retail customers that have any quantities of the two recalled lots should stop distribution and return any unsold units to their wholesaler.
Consumers with questions regarding this recall can contact Taro by calling 1-866-705-1553 or by e-mail at TaroPVUS@taro.com.