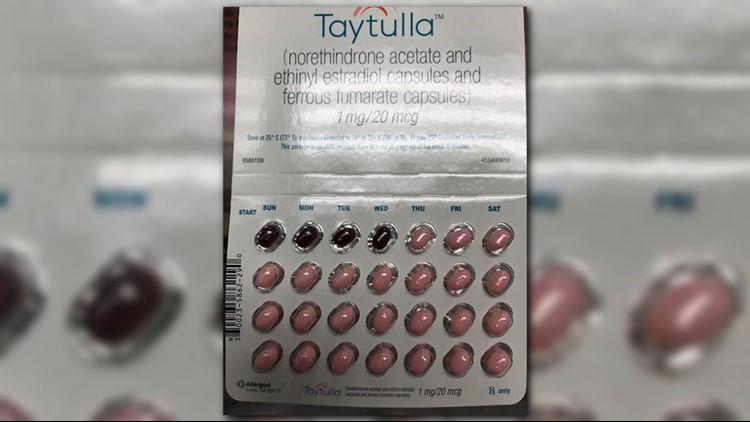
Allergan is recalling almost 170,000 sample packs of birth control pills because placebo and active capsules were packaged out of order, heightening the risk of unwanted pregnancy.
The drugmaker on Tuesday said the physician sample packs of Taytulla were intended to have 24 active pink pills, followed by four inactive maroon capsules, taken daily for 28 days. The picture below is how the packet is supposed to look.
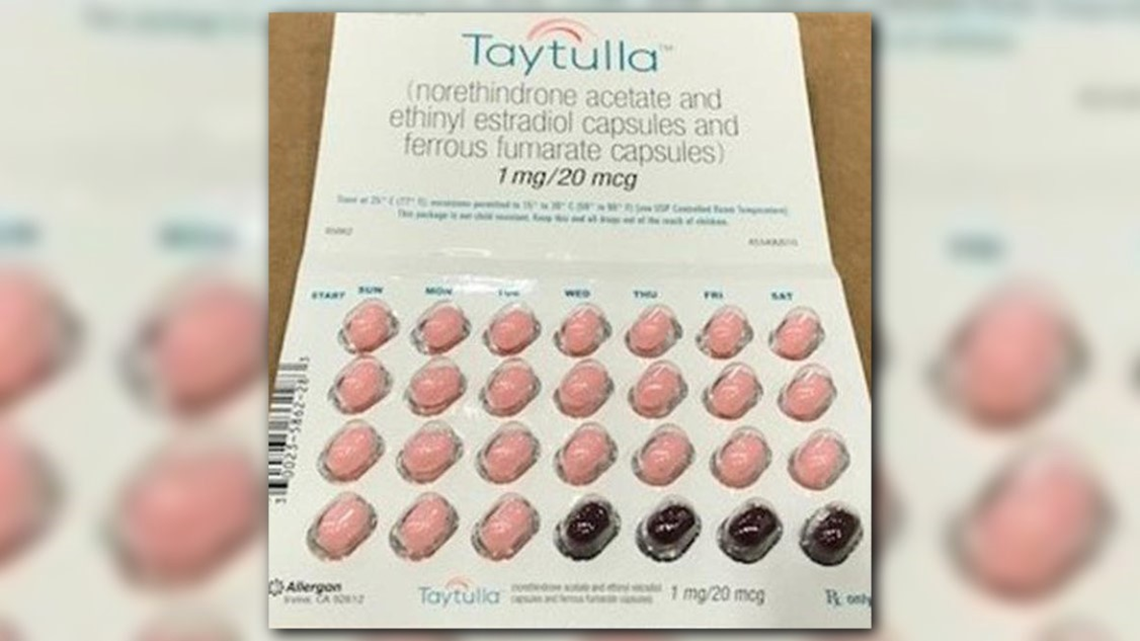
An Allergan spokesperson confirmed to CBS MoneyWatch that there were 168,768 units in the lot involved. "The packing error was detected in a single pack," the spokesperson added.
The packs are marked with the lot number 5620706 and carry a May 2019 expiration date.
The company issued its nationwide recall after a physician reported a sample pack containing the first four days of therapy as having the non-hormonal placebo pills instead of the actives ones, Allergan said in a statement.
"The reversing of the order may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order," according to the recall posted by the U.S. Food and Drug Administration. "If patients have concerns regarding the possibility of an unintended pregnancy they should consult their physician."
Allergan said it was notifying consumers by letter of the potential trouble. Shares of the publicly traded company fell almost 2% on Tuesday to about $151.
► Make it easy to keep up to date with more stories like this. Download the WFMY News 2 App: Apple Users, Android Users